Which of the Following Contains the Greatest Number of Molecules
A mole of a chemical substance represents. 2 State which one of.

Which Of The Following Contains The Greatest Number Of Atoms Youtube
2 moles of N20 5 holes of Xe 2 mole of CO2 4 moles of CO Question 18 2.

. 3 20 moles of Ar. Which of the following samples contains the greatest number of molecules. The Cullinan diamond was the.
For example H2O is water has 2 different kinds of atoms which are H and O. Question 17 2 pts Which of the following samples contains the greatest number of atoms. A million0 M MgCl2 by skill of way of fact it releases 1mol Mg2 and a couple of mol Cl- according to liter for a entire of three mol according to liter.
D 66 g of carbon dioxide. Thus the substance with the greatest number of moles will have the. Chemistry questions and answers.
1 mole of all substance contain equal number of molecules. A i b i. PH 736 PaO2 59 CO2 45 HCO3 22.
So that is 2 moles of atoms per mole of NaCl. We know that one mole of any substance contains 6022 10 23 molecules irrespective of the nature of the substance. 5 20 million O2.
K X 2 S O X 4 has 2 moles of potassium 1 mole of S and 4 moles of O per mole. Sugar is C12 H22 O11 the molecular weight or molar. I 2 moles of H2O.
N m M. Thus 224 liters have a 6022 1023 number of particles. Diamond is one form of elemental carbon.
1 g sulfur S8. Thus one mole contains 2. His current ABG test results indicate.
There is the answer. 05 M NaCl components. Total number of atoms 00092 x 6022 x 10 23 0054 x 10 23 atoms Molecular mass of Water H 2 O is 18gmol There are 3 atoms in the molecule So 1g of water means.
Which of the following will contain the greatest number of molecules at 300K and 1 atm pressure. One mole has a 6022 1023 number of particles. Iii 60221023 molecules of water.
Each molecule of nitrogen contains two atoms of each. 2g H 2 2 2 1 mole of. 1 g phosphorus P4 B.
1 State which one of the following contains the greatest number of molecules. He is having complications that are preventing him from being discharged to a rehabilitation facility. Which of the following samples contains the greatest number of atoms.
Iv 120441025 molecules of water. 1 g nitrogen N2 D. Maximum number of molecules are found in 1 gram hydrogen.
An engagement ring contains a diamond weighing 125 carats. What is the name of the molecule that has the greatest number of different kinds of atoms. 1 g of CH 3 Cl.
So substance with more number of moles have more number of molecules. 2 5g of He. 7g N 2 7 28 025 moles of molecules.
Which of the following correctly represents 360 g of water. NaCl contains 2 ions per formula unit NH 4 Cl contains 2 ions per formula unit AlCl 3 contains 4 ions per formula unit and MgNO 3 2 contains 3 ions per formula unit. 1 g chlorine Cl2 C.
Which of the following contains the greatest number of atoms. Geeze NaCl has 1 mole of Na and 1 mole of Cl per mole of NaCl. A 3g of hydrogen.
Which of the following statements concerning Avogadros number is correct. B 32 g of oxygen. A 1 mole of S8 molecules B 2 moles of P4.
Convert 10 lbs to grams divide by the molecular weight of sugar multiply by avogadros number multiply by 12. C 36g of water. Ii 20 moles of water.
Number of moles Given mass of substance Molecular mass of substance. 602 x 1023 chemical particles of the substance. 4 01 mole of Fe.
Number of moles. 1 100g of Pb.
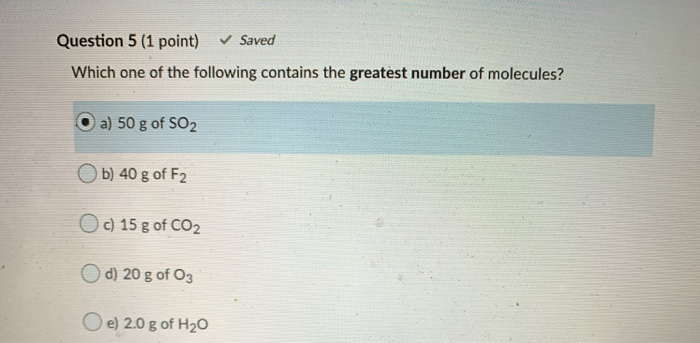
Solved Question 5 1 Point Saved Which One Of The Chegg Com

Solved Which Sample Contains The Largest Number Of Chegg Com

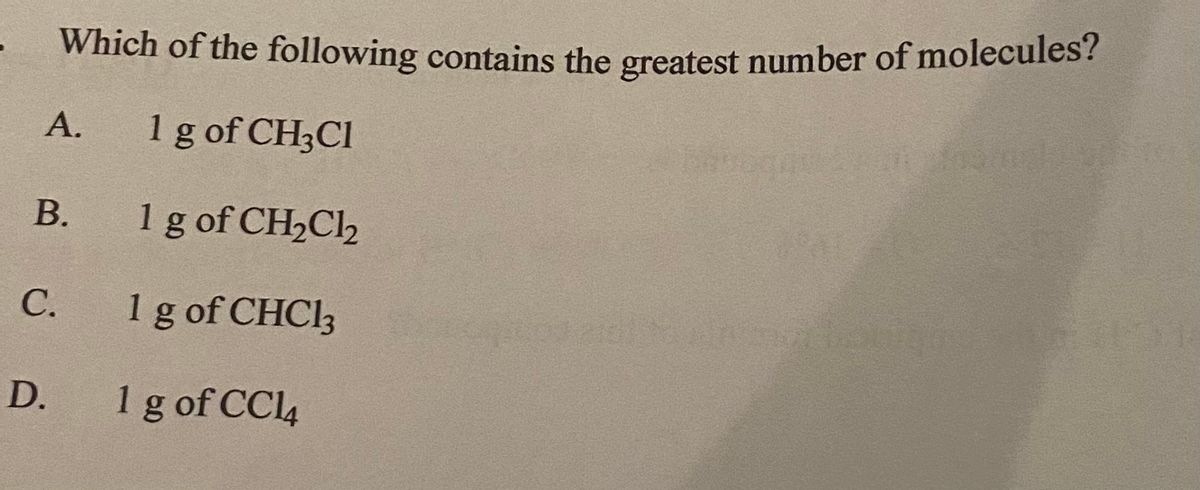
No comments for "Which of the Following Contains the Greatest Number of Molecules"
Post a Comment